
Prostate cancer is the most commonly diagnosed cancer among Canadian men. However, with improved testing and better treatment options, the number of deaths from the disease has been declining over the last several years.
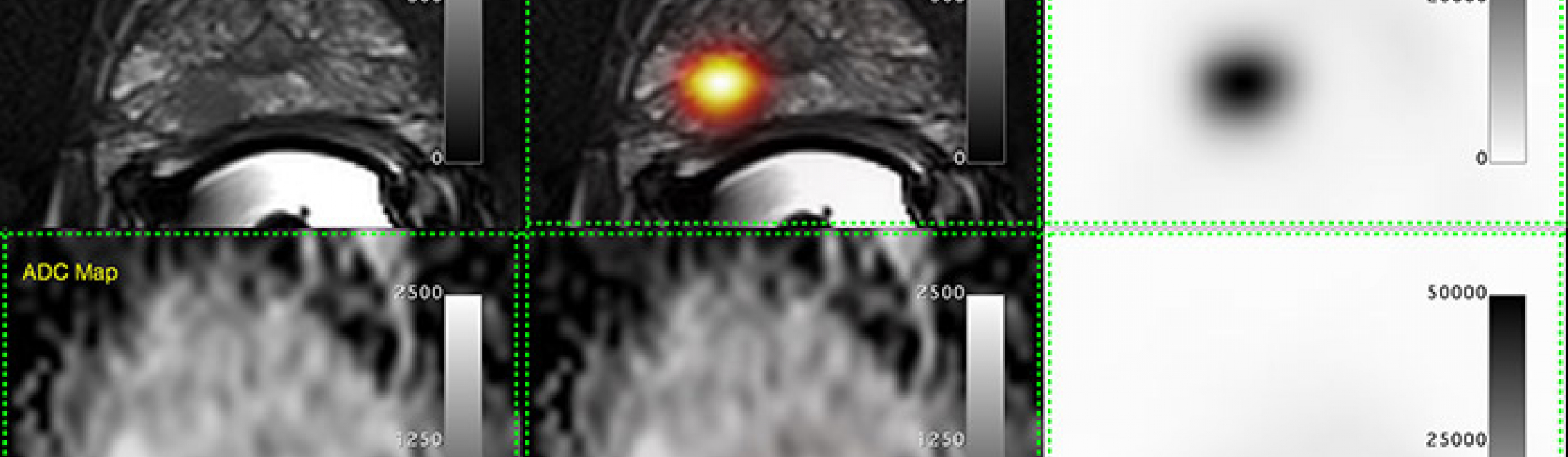
Scientists at Lawson Health Research Institute are working to continue this trend by testing improved prostate cancer imaging using a new molecule. Known as a Prostate Specific Membrane Antigen (PSMA) probe, the new molecule is used in Positron Emissions Tomography (PET) scans. The probe targets PSMA, a unique molecule on prostate cancer cells, to provide highly specific images for better diagnosis and management of patient disease.
PET probes are used in imaging to correctly diagnose cancer. The probes are injected into a patient where they spread to identify sites of disease. The most common PET probes are suitable for many types of cancer, but are not as sensitive in identifying prostate cancer. PSMA probes provide higher accuracy by targeting PSMA molecules, which are highly over-expressed on prostate cancer cells.
PSMA probes are gaining popularity across the globe. This specific probe is a molecule called 18F-DCFPyL and was developed by Dr. Martin Pomper at the John Hopkins Hospital in Baltimore. Dr. Pomper, also a Scientific Advisor to Lawson’s prostate imaging team, worked in collaboration with Canada’s Centre for Probe Development and Commercialization (CPDC) to bring the probe to our nation.
Lawson’s Canadian Institutes of Health Research (CIHR) Team in Image Guidance for Prostate Cancer gained early access to the PSMA probe due to a history of close collaboration with Dr. Pomper and the CPDC. Marking the first time a PSMA probe has been used in Canada, the team captured PET/MRI and PET/CT images from a 64-year-old prostate cancer patient on March 18, 2016 at St. Joseph’s Hospital.
“This is a tremendous step forward in the management of prostate cancer,” said Dr. Glenn Bauman, a Lawson scientist and Radiation Oncologist at London Health Sciences Centre. “PSMA probes have the potential to provide increased accuracy and detection which leads to better, personalized treatment.”
Lawson plans to study the probe with an additional 20 men over the next two years as part of an ongoing clinical trial funded by the Ontario Institute for Cancer Research (OICR). Lawson scientists are working with researchers across Ontario to develop other clinical trial protocols that will use 18F-DCFPyL to measure responses to drug treatments and to evaluate men with suspected recurrence of prostate cancer after radiotherapy.
“The goal of these studies it to establish the value of PSMA probes, particularly18F-DCFPyL, and provide evidence to support the use of these probes in routine clinical care,” said Dr. Bauman.
Donor funding through London Health Sciences Foundation was one catalyst in this research, providing initial funding to hire Research Associate, Catherine Hildebrand, who set up citywide cancer imaging workshops and helped the team prepare successful grant applications to secure key funding from CIHR and OICR.