
Treatment-resistant depression is a severe form of illness that does not respond to traditional therapies like medication and counselling. It is particularly common in patients with depression from bipolar disorder who are left with limited treatment options.
“There are some mental illnesses that can become resistant to therapy, similar to how infections, for example, can become resistant to antibiotics,” explains Dr. Amer Burhan, researcher at Lawson Health Research Institute and neuropsychiatrist at St. Joseph’s Health Care London. “Patients with those illnesses need more options.”
Brain stimulation is a field that holds promise for this patient population.
“When people are in a state of depression, research shows their brain networks are not functioning properly,” says Dr. Burhan. “Brain stimulation aims to stimulate neurons in the brain to correct activity and improve patient outcomes.”
Through involvement in a national clinical trial, Dr. Burhan and his research team at Lawson are offering new hope with a treatment called magnetic seizure therapy (MST). The study is the first randomized controlled trial to examine the efficacy of MST for patients with treatment-resistant depression as a result of bipolar disorder.
For years electroconvulsive therapy (ECT), one form of brain stimulation, has been the gold standard for patients with treatment-resistant depression. ECT uses an electric field to induce a seizure that provides a therapeutic benefit by stimulating the brain. But while ECT is effective, many patients opt out of treatment due to stigma surrounding the therapy and its potential for cognitive side effects like disorientation and amnesia.
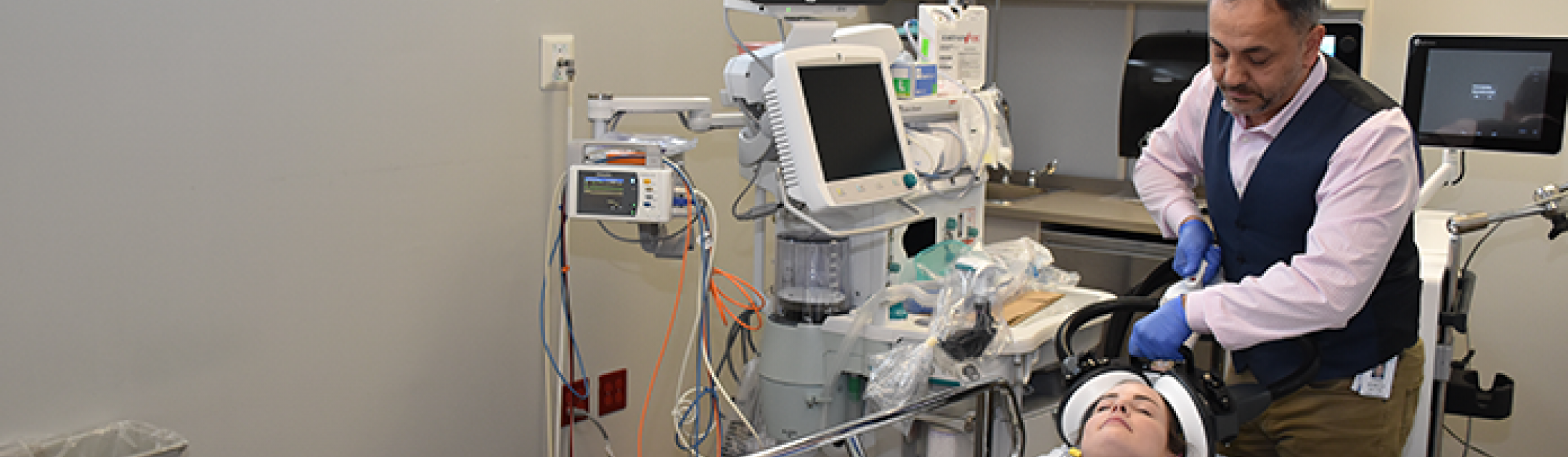
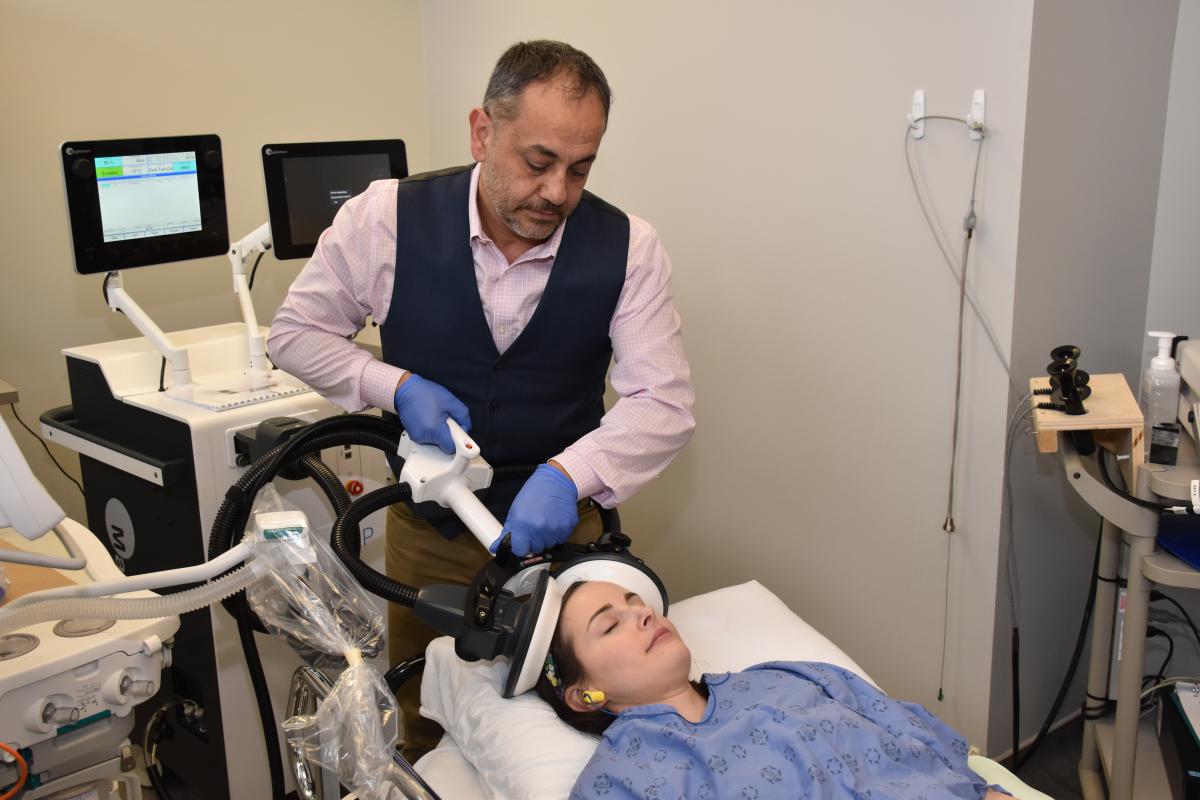
MST has emerged as a promising alternative. MST works in a similar way to ECT but uses a focused magnetic field as opposed to electricity. As a result, MST induces a more focused seizure to reduce the risk of cognitive side effects.
“Magnetic seizure therapy has already been shown as a promising treatment for major depressive disorder or unipolar depression,” explains Dr. Amer Burhan, local site lead for the clinical trial. “For the first time, we’re studying how effective the treatment is for depression as a result of bipolar disorder and whether it can reduce the risk of cognitive side effects associated with electroconvulsive therapy.”
The clinical trial is being led by the Centre for Addiction and Mental Health (CAMH) and will also be offered through Lawson and University of British Columbia (UBC) Hospital. Lawson researchers will invite approximately 30 eligible patients with treatment-resistant depression from bipolar disorder to participate in the trial at Parkwood Institute, a part of St. Joseph’s Health Care London.
Eligible patients will be randomized to receive either ECT or MST. Patients will be offered support throughout the study with the goal of improving patient outcomes in both groups. Patient outcomes will be compared to study the whether MST is effective and whether it is associated with reduced cognitive side effects.
MST will be delivered under anesthesia in 12 to 20 sessions. Sessions will last 10 to 15 minutes each with 60 to 90 minutes of recovery time.
“Magnetic seizure therapy holds promise of one day replacing electroconvulsive therapy as the gold standard for treatment-resistant depression, but we need to learn more about where it fits in our toolbox of potential treatments,” says Dr. Burhan.
If proven as a viable first-line treatment, MST would be very easy to implement in existing ECT clinics. MST would therefore be readily available to patients in need.
“We are on the leading edge of the field of brain stimulation for treatment-resistant depression in collaboration with CAMH and UBC,” says Dr. Burhan. “Our goal is to continue informing the care process through clinically-relevant research that serves patients, medical professionals and the public.”
Those who would like more information about the trial can email @email or @email.