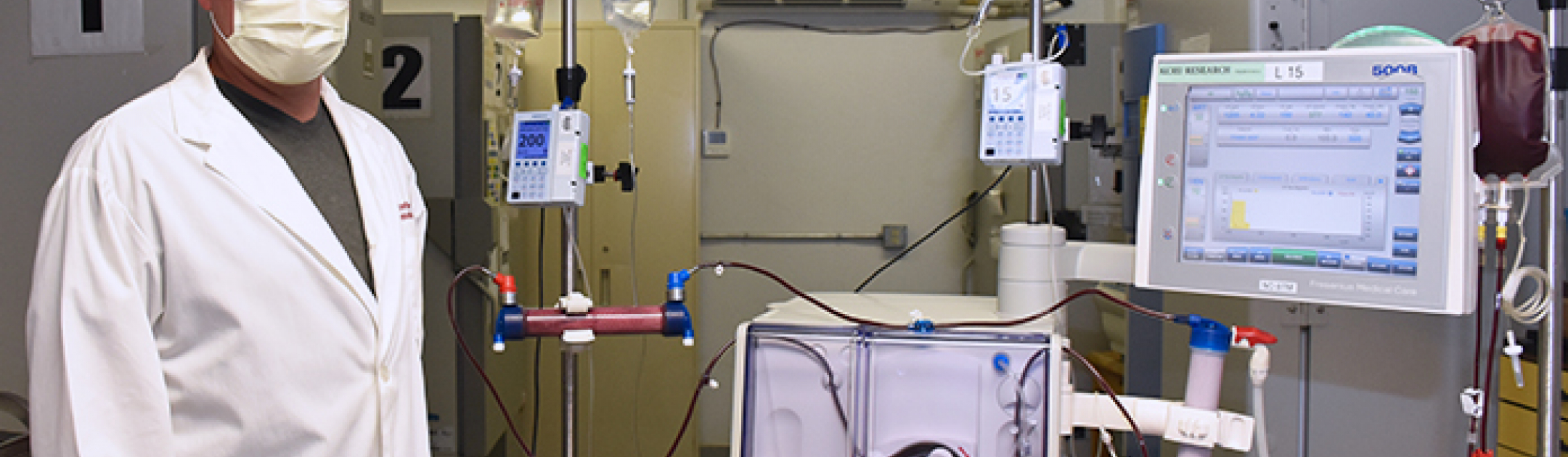

As part of a randomized controlled trial, a team from Lawson Health Research Institute is the first in the world to treat a patient with COVID-19 using a modified dialysis device. The device gently removes a patient’s blood, modifies white blood cells and returns them to fight hyperinflammation. It is being tested with critically ill patients at London Health Sciences Centre (LHSC).
Evidence suggests that COVID-19 causes a heightened immune response, termed a ‘cytokine storm,’ in the most severely ill patients. Treatment options to address this hyperinflammatory state are currently limited and there are concerns about global drug shortages.
“Working in the intensive care unit (ICU), I was aware that more treatment options were needed in the fight against COVID-19,” says Dr. Chris McIntyre, lead researcher, Lawson Scientist and LHSC Nephrologist. “This led to the idea of treating a patient’s blood outside of the body. We could reprogram white blood cells associated with inflammation to alter the immune response.”
The research uses a modified version of a standard dialyzer called an extracorporeal leukocyte modifying device. It gently removes blood in a much slower circuit than standard dialysis. Through a process using specific levels of biochemical components, it targets and transforms white blood cells associated with inflammation before releasing them back into circulation. The hope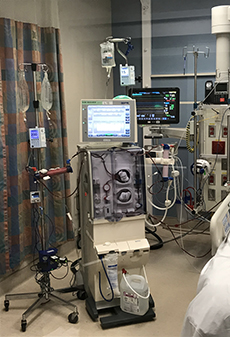
The clinical trial will include up to 40 critically ill patients with COVID-19 at LHSC’s Victoria Hospital and University Hospital. Research participants will be randomized to receive either standard supportive care or standard supportive care in combination with this novel treatment. The research team will compare patient outcomes to determine if the treatment is effective.
“The ultimate goal is to improve patient survival and lessen their dependency on oxygen and ventilation,” explains Dr. McIntyre. “If effective, it’s possible that this treatment could be combined with other therapies. For example, this could be used to modulate inflammatory consequences while an antiviral drug is used to reduce the viral load.”
Led by Lawson’s Kidney Clinical Research Unit, this new trial was accelerated from initial conception to treatment of the first patient in only 40 days. It represents an important research collaboration with a multidisciplinary team. The trial is leveraging insights gained from another local study led by Dr. Douglas Fraser which is analyzing blood samples from COVID-19 patients at LHSC to better understand the cytokine storm.
“We’re identifying which cytokines or biomarkers are important to the hyperinflammatory response seen in COVID-19 patients,” says Dr. Fraser, Scientist at Lawson and Paediatric Critical Care Physician at LHSC. “With the knowledge we’re gaining, we can study a patient’s blood to determine whether this extracorporeal treatment is making a difference.”
If successful, the treatment also has potential to be used with other conditions like sepsis.
