
As we continue to live with a COVID-19 pandemic, patients will need good treatment options.
Hospital researchers in London, Ontario, through Lawson Health Research Institute, are testing treatment options for patients who have been hospitalized from a COVID-19 infection, often for severe symptoms.
During the peaks of the COVID-19 pandemic, there were concerns of a global drug shortage when it came to IV sedatives for patients needing ventilation. Through funding from the Government of Ontario’s COVID-19 Rapid Research Fund, a team of Ontario researchers studied whether inhaled sedatives could replace those that are delivered through IV.
“In addition to easing the burden on IV stocks, the inhaled sedatives have additional benefits,” says Dr. Marat Slessarev, a Critical Care Physician at LHSC. “They may reduce inflammation in the lungs and shorten the duration of sedation because they are eliminated from the body faster than IV sedatives.”
Dr. Slessarev, also a Scientist at Lawson, adds that using inhaled sedatives could also be safer for severe COVID-19 patients, who in many cases are on a ventilator for a long time. “When using IV sedatives, they can accumulate and metabolize with accumulation. This can leave the patient with the potential of developing kidney issues because it is hard to dispel them quickly.”

Dr. Marat Slessarev, Critical Care Physican at LHSC and Lawson Scientist
To date, around 800 patients across ten hospital sites have been enrolled in this trial, with the hopes of getting more hospital sites on board as the clinical trials continue.
A majority of people with severe COVID-19 infections in critical care end up developing sepsis, which is a potentially life-threatening condition that occurs when the body's response to an infection damages its own tissues.
“An inflammatory response is what the body uses to fight an infection, but sometimes it is more than necessary and it causes damage to the organs and the body,” explains Dr. Claudio Martin, a Physician in the Intensive Care Unit (ICU) at London Health Sciences Centre (LHSC). “This is why a patient with a severe COVID-19 infection caused by a virus can get sepsis.”
Dr. Martin, who is also an Associate Scientist at Lawson, began studying the use of a human protein called Annexin A5 as a potential treatment for COVID-19 patients with sepsis. “It can work in two ways. The annexin may coat injured cells and reduce the inflammatory response. Or, the injured cells and exposed proteins trigger the clotting mechanism in the body. Those with COVID-19 could get blood clots in the brain and the lungs, and using annexin may improve their outcomes.”

Dr. Claudio Martin, Critical Care Physician at LHSC and Lawson Scientist
Currently, a clinical trial using Annexin A5 on severe COVID-19 patients is underway at LHSC to look at the efficacy of using this human protein.
The COVID-19 virus is also known to cause respiratory failure, prompting Dr. Jim Lewis, Respirologist at St. Joseph’s Health Care London and Scientist at Lawson, to investigate the use of pulmonary surfactant as a potential treatment for these patients.
Bovine Lipid Extract Surfactant Suspension (BLES) is a pulmonary surfactant manufactured in London, Ontario. It’s currently used worldwide to help improve lung function in premature babies. Now, it is being studied with COVID-19 patients experiencing respiratory failure.

Dr. Jim Lewis, Repirologist at St. Joseph's Health Care London and Lawson Scientist
“We know that patients who have injury to their lung and require ventilation have inflammation in the airways of their lungs. Surfactant is a homogeneous layer of lipids and proteins that line the lungs to allow us to breath with minimal effort,” explains Dr. Lewis. “If we gave these patients surfactant as soon as possible after they were put on a mechanical ventilator, it may have some benefit in improving their outcome and getting them off the ventilator sooner.”
Dr. Lewis and his team conducted a clinical trial using pulmonary surfactant with ten critically ill COVID-19 patients and initial results have shown patient safety and efficacy.
Another team of hospital researchers turned to using modified dialysis machines to offer treatment options to those with severe COVID-19 symptoms. Nephrologist at LHSC, Dr. Chris McIntyre, was the first in the world to modify a dialysis device to treat a patient with COVID-19. The device gently removes a patient’s blood, modifies white blood cells, and returns them to fight hyperinflammation.
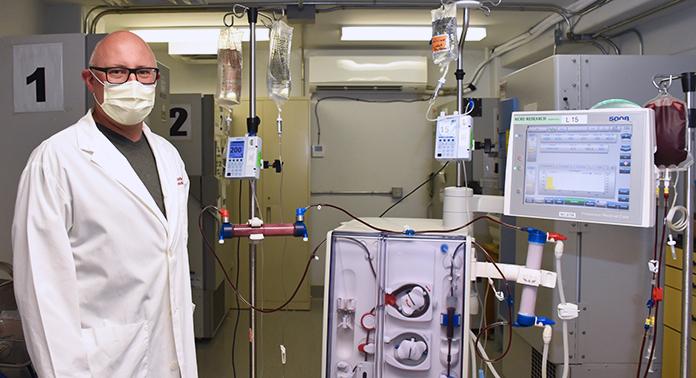
Dr. Chris McIntyre, Nephrologist at LHSC and Lawson Scientist
“It was quick. We went from the initial idea to the approvals, creating the device and appointing the first patient within 40 days,” says Dr. McIntyre who is also a Scientist at Lawson. “After the first 12 patients, we found that those treated with the device needed significantly less drugs to maintain their blood pressure. We were able to deliver the treatment for all the patients – none of the treatments failed and we had no safety issues.”
Dr. McIntyre is now looking into using this form of therapy for chronic dialysis patients to help modify organ injury.